Background: Mosunetuzumab is a CD20xCD3 T-cell engaging bispecific antibody that redirects T cells to eliminate malignant B cells. In a pivotal Phase II study (NCT02500407), mosunetuzumab demonstrated a high complete response (CR) rate with a manageable safety profile in patients with relapsed/refractory (R/R) follicular lymphoma (FL) and ≥2 prior lines of therapy (Budde et al. Lancet Oncol 2022). Mosunetuzumab is a fixed-duration treatment that can be administered in an outpatient setting. Here, we present updated data for patients with R/R FL and ≥2 prior lines of therapy, after 3 years of follow-up.
Methods: Eligible patients with R/R FL Grade (Gr) 1-3a and ≥2 prior therapies received intravenous mosunetuzumab in 21-day cycles with step-up dosing in Cycle (C) 1 (C1 Day [D] 1, 1mg; C1D8, 2mg; C1D15/C2D1, 60mg; C3D1 and onwards, 30mg). Hospitalization for treatment was not required. Patients achieving a CR by C8 completed treatment without additional cycles; patients with a partial response or stable disease received a total of 17 cycles. The primary endpoint was CR rate as determined by an Independent Review Committee (as best response; Cheson 2007 criteria). Duration of response (DOR), duration of complete response (DOCR), progression-free survival (PFS), event-free survival (EFS), and safety were secondary endpoints. Time to next treatment (TTNT), response to retreatment, biomarkers of minimal residual disease (MRD), and circulating B-cell counts were exploratory endpoints.
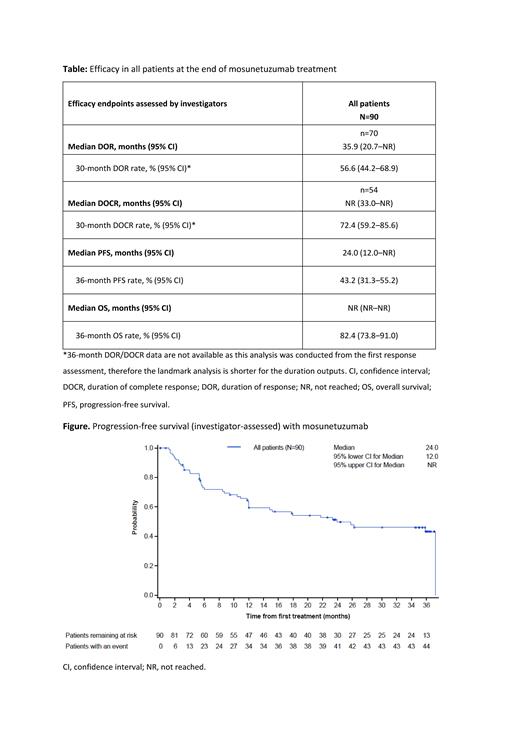
Results: Ninety patients with R/R FL were enrolled. As of May 2, 2023, median time on study was 37.4 (range: 2.0-48.0) months. Investigator-assessed best overall response and CR rates were 77.8% (95% CI: 67.8-85.9) and 60.0% (95% CI: 49.1-70.2), respectively. The median DOR was 35.9 months (95% CI: 20.7-not reached [NR]). Median DOCR was NR (95% CI: 33.0-NR); the estimated 30-month DOCR rate was 72.4% (95% CI: 59.2-85.6) ( Table). Three years after the end of treatment, 57.1% of 70 responding patients were alive and disease progression-free. Median PFS was 24.0 months (95% CI: 12.0-NR) ( Figure). The median TTNT was 37.3 months (95% CI: 18.0-NR); estimated EFS at 36 months was 51.8% (95% CI: 40.8-62.8). After initial mosunetuzumab treatment, 34/90 (37.8%) patients had received a new anti-lymphoma therapy, including 33/90 (36.7%) patients who received a new systemic treatment (8/33 [24.2%] of these patients received chimeric antigen receptor T-cell therapy); 8/90 (8.9%) had radiotherapy; 2/90 (2.2%) had excision of tumor; 2/90 (2.2%) had an allogeneic stem cell transplant; and 1/90 (1.1%) had an autologous stem cell transplant. Five patients received retreatment with mosunetuzumab, 3/5 of these patients had a CR. No new cytokine release syndrome (CRS) events, serious, or Gr ≥3 adverse events (AEs) were reported since the previous analysis (median follow-up of 28.3 months; Bartlett et al. ASH 2022). CRS events occurred in 44.4% of patients and 2.2% were Gr 3/4 in severity; all CRS events resolved. Overall AEs and serious AEs were comparable to the previous analysis (Bartlett et al. ASH 2022); febrile neutropenia was not reported. Forty-six (51.1%) patients experienced Gr 3/4 AEs related to mosunetuzumab; the rate of AEs leading to discontinuation was low (4.4%). Peripheral blood B-cell depletion following treatment with mosunetuzumab occurred in all patients, and recovery was observed after a median of 18 months following the end of treatment in patients with a sustained response using follow-up samples. MRD kinetics following mosunetuzumab therapy will be presented.
Conclusions: In this updated analysis, with a median follow-up of 37.4 months, durable responses continued to be observed with fixed-duration mosunetuzumab in patients with R/R FL. The manageable safety profile was consistent with previous reports. Evidence of B-cell recovery was observed after a median of 18 months following the end of treatment.
Disclosures
Schuster:Pharmacyclics: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Gilead: Research Funding; Janssen Research & Development: Research Funding; Merck: Research Funding; Nordic Nanovector: Other: Scientific Advisory Committee; Genentech: Consultancy; Acerta: Consultancy; Novartis: Consultancy, Research Funding. Sehn:Merck: Consultancy; AbbVie: Consultancy; Amgen: Consultancy; Incyte: Consultancy; Genentech/Roche: Consultancy; AstraZeneca: Consultancy; BeiGene: Consultancy; BMS/Celgene: Consultancy; Kite/Gilead: Consultancy; Janssen: Consultancy; Seattle Genetics: Consultancy; Roche/Genentech: Research Funding; Teva: Research Funding. Bartlett:ADC Therapeutics, Foresight Diagnostics, Kite, F. Hoffmann-La Roche Ltd / Genentech, Inc., Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics, Autolus, BMS/Celgene, Forty Seven, Gilead/Kite Pharma, Janssen, Merck, Millennium, Pharmacyclics, F. Hoffmann-La Roche Ltd / Genentech, Inc., Seattle Genetics: Research Funding; Washington University School of Medicine: Current Employment. Matasar:Juno: Consultancy; Genentech, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seagen: Honoraria, Other: stipends; Regeneron: Honoraria, Other: Stipends; Kite: Honoraria, Other: Stipends; AstraZeneca: Honoraria, Other: Stipend; Merck: Current equity holder in private company; ADC Therapeutics: Consultancy, Honoraria, Other: Stipend; Immunovaccine Technologies: Honoraria; Takeda: Consultancy, Honoraria; Epizyme: Other: Stipends; Bayer: Consultancy, Honoraria, Research Funding; Pharmacyclics: Honoraria, Research Funding; BMS: Honoraria, Other: Stipend; Celegene: Honoraria, Other: Stipends; Teva: Consultancy; Janssen: Honoraria, Research Funding; Immunovaccine Technologies: Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Assouline:BeiGene: Consultancy; Novartis Canada: Research Funding; AbbVie: Honoraria; Janssen: Honoraria; AstraZeneca: Honoraria; Roche-Genentech: Honoraria; Ipsen: Consultancy; Gilead: Honoraria; Palladin: Honoraria. Giri:Royal Adelaide Hospital: Current Employment. Kuruvilla:Karyopharm: Other: DSMB; F. Hoffmann-La Roche Ltd, Astra Zeneca, Merck: Research Funding; Abbvie, Amgen, AstraZeneca, BMS, Genmab, Gilead, Incyte, Janssen, Merck, Novartis, Pfizer, F. Hoffman-La Roche Ltd, Seattle Genetics: Honoraria; Abbvie, BMS, Gilead, Merck, F. Hoffmann-La Roche Ltd, Seattle Genetics: Consultancy. Shadman:Mustang Bio, BMS, Pharmacyclics, Genentech, Inc., AbbVie,TG Therapeutics, BeiGene, AstraZeneca, Genmab, MorphoSys/Incyte, Vincerx: Research Funding; AbbVie, Genentech, Inc., AstraZeneca, Pharmacyclics, BeiGene, BMS, MorphoSys/Incyte, Kite, Eli Lilly, Genmab, Mustang Bio, Regeneron, ADC therapeutics, Fate Therapeutics, Janssen, MEI Pharma: Consultancy; regeneron: Consultancy, Research Funding; Lilly: Consultancy; pharmacyclics: Consultancy, Research Funding; beigene: Consultancy, Research Funding; abbvie: Consultancy; genentech: Consultancy, Research Funding. Cheah:F. Hoffmann-La Roche Ltd, Janssen, Gilead, AstraZeneca, Lilly, TG therapeutics, Beigene, Novartis, Menarini, Daizai, Abbvie, Genmab. BMS: Honoraria; BMS, F. Hoffmann-La Roche Ltd, Abbvie; MSD, Lilly: Research Funding; F. Hoffmann-La Roche Ltd, Janssen, Gilead, AstraZeneca, Lilly, TG therapeutics, Beigene, Novartis, Menarini, Daizai, Abbvie, Genmab. BMS: Consultancy. Dietrich:F. Hoffmann-La Roche Ltd, Kite, Gilead and BeiGene: Honoraria; University Hospital of Heidelberg, Germany: Ended employment in the past 24 months; University Hospital of Duesseldorf, Germany: Current Employment. Ku:Antengene, Genor BioPharma, F. Hoffmann-La Roche Ltd: Consultancy; Clinical Haematologist St Vincent's Hospital, Melbourne: Current Employment. Nastoupil:Gilead Sciences/Kite Pharma: Honoraria, Research Funding; DeNovo: Honoraria; Caribou Biosciences: Honoraria, Research Funding; Regeneron: Honoraria; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; AstraZeneca: Honoraria; ADC Therapeutics: Honoraria; Daiichi Sankyo: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; AbbVie: Honoraria. Wei:Genentech, Inc.: Current Employment; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company; F. Hoffmann-La Roche Ltd: Patents & Royalties. Yin:F. Hoffmann-La Roche Ltd / Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. To:Genentech, Inc.: Current Employment. Huang:F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Kwan:F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company; F. Hoffmann-La Roche Ltd / Genentch, Inc.: Current Employment. Penuel:Genentech, Inc. / F. Hoffman-La Roche Ltd: Current Employment, Current holder of stock options in a privately-held company. Budde:Novartis, Gilead, F. Hoffmann-La Roche Ltd, BeiGene, Genentech, Inc.: Consultancy; ADC Therapeutics: Consultancy; MustangBio: Research Funding; Merck: Research Funding; AstraZeneca: Consultancy, Research Funding; Amgen: Research Funding; Roche: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal